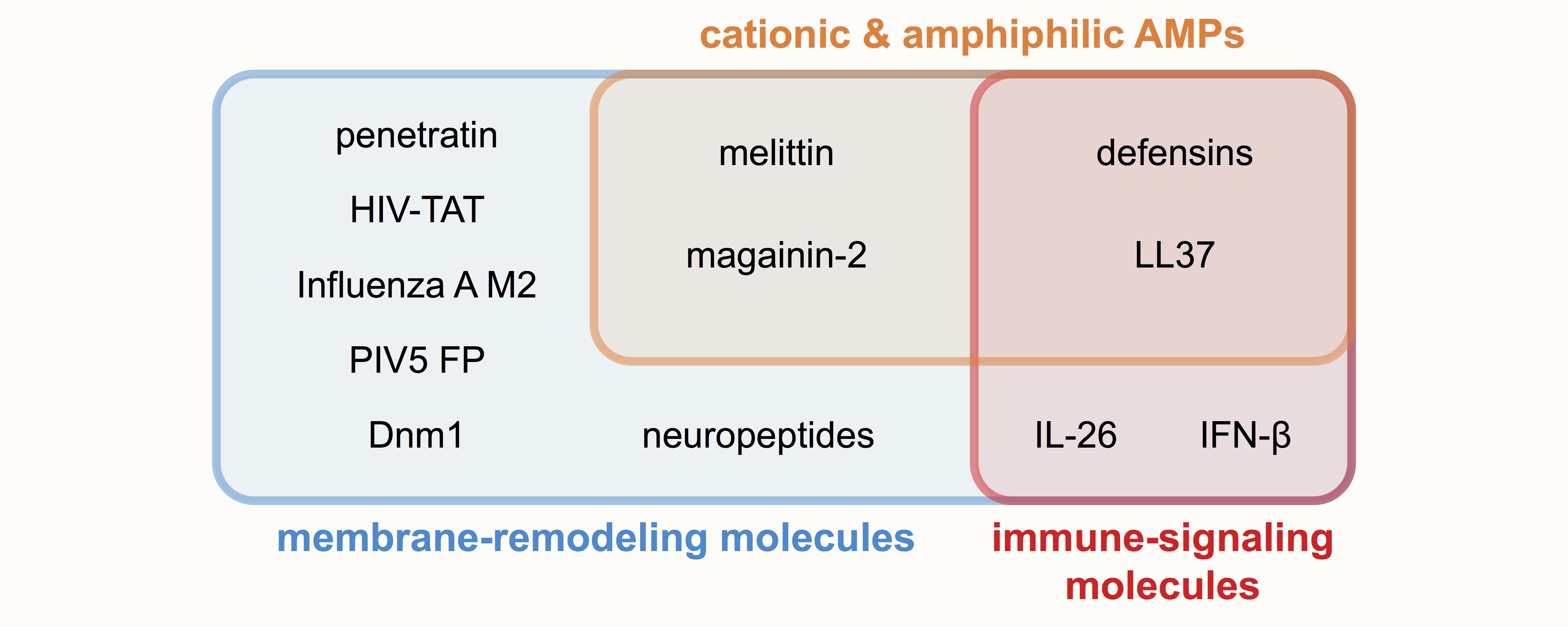
 Membrane-remodeling peptides and proteins have diverse primary functions
Membrane-remodeling peptides and proteins have diverse primary functions
Machine Learning Antimicrobial Peptide Sequences: Some Surprising Variations on the Theme of Amphiphilic Assembly
Abstract
Antimicrobial peptides (AMPs) collectively constitute a key component of the host innate immune system. They span a diverse space of sequences and can be α-helical, β-sheet, or unfolded in structure. Despite a wealth of knowledge about them from decades of experiments, it remains difficult to articulate general principles governing such peptides. How are they different from other molecules that are also cationic and amphiphilic? What other functions, in immunity and otherwise, are enabled by these simple sequences? In this short review, we present some recent work that engages these questions using methods not usually applied to AMP studies, such as machine learning. We find that not only do AMP-like sequences confer membrane remodeling activity to an unexpectedly broad range of protein classes, their cationic and amphiphilic signature also allows them to act as meta-antigens and self-assemble with immune ligands into nanocrystalline complexes for multivalent presentation to Toll-like receptors.