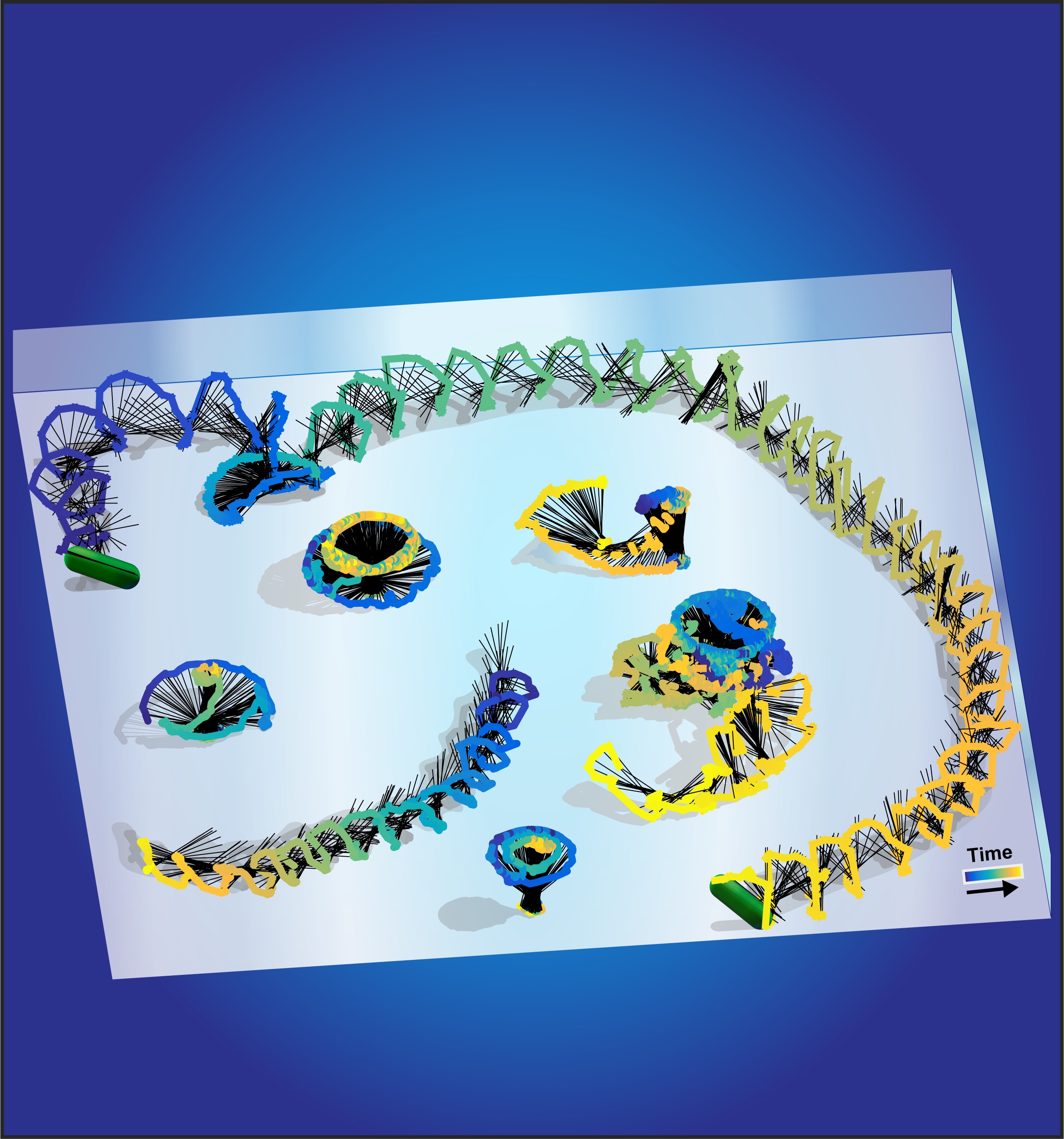
 Diverse motility modes of Pseudomonas near a surface.
Diverse motility modes of Pseudomonas near a surface.
High-Speed "4D" Computational Microscopy of Bacterial Surface Motility
Abstract
Bacteria exhibit surface motility modes that play pivotal roles in early-stage biofilm community development, such as type IV pili-driven “twitching” motility and flagellum-driven “spinning” and “swarming” motility. Appendage-driven motility is controlled by molecular motors, and analysis of surface motility behavior is complicated by its inherently 3D nature, the speed of which is too fast for confocal microscopy to capture. Here, we combine electromagnetic field computation and statistical image analysis to generate 3D movies close to a surface at 5 ms time resolution using conventional inverted microscopes. We treat each bacterial cell as a spherocylindrical lens and use finite element modeling to solve Maxwell’s equations and compute the diffracted light intensities associated with different angular orientations of the bacterium relative to the surface. By performing cross-correlation calculations between measured 2D microscopy images and a library of computed light intensities, we demonstrate that near-surface 3D movies of Pseudomonas aeruginosa translational and rotational motion are possible at high temporal resolution. Comparison between computational reconstructions and detailed hydrodynamic calculations reveals that P. aeruginosa act like low Reynolds number spinning tops with unstable orbits, driven by a flagellum motor with a torque output of 2 pN μ. Interestingly, our analysis reveals that P. aeruginosa can undergo complex flagellum-driven dynamical behavior, including precession, nutation, and an unexpected taxonomy of surface motility mechanisms, including upright-spinning bacteria that diffuse laterally across the surface, and horizontal bacteria that follow helicoidal trajectories and exhibit superdiffusive movements parallel to the surface.